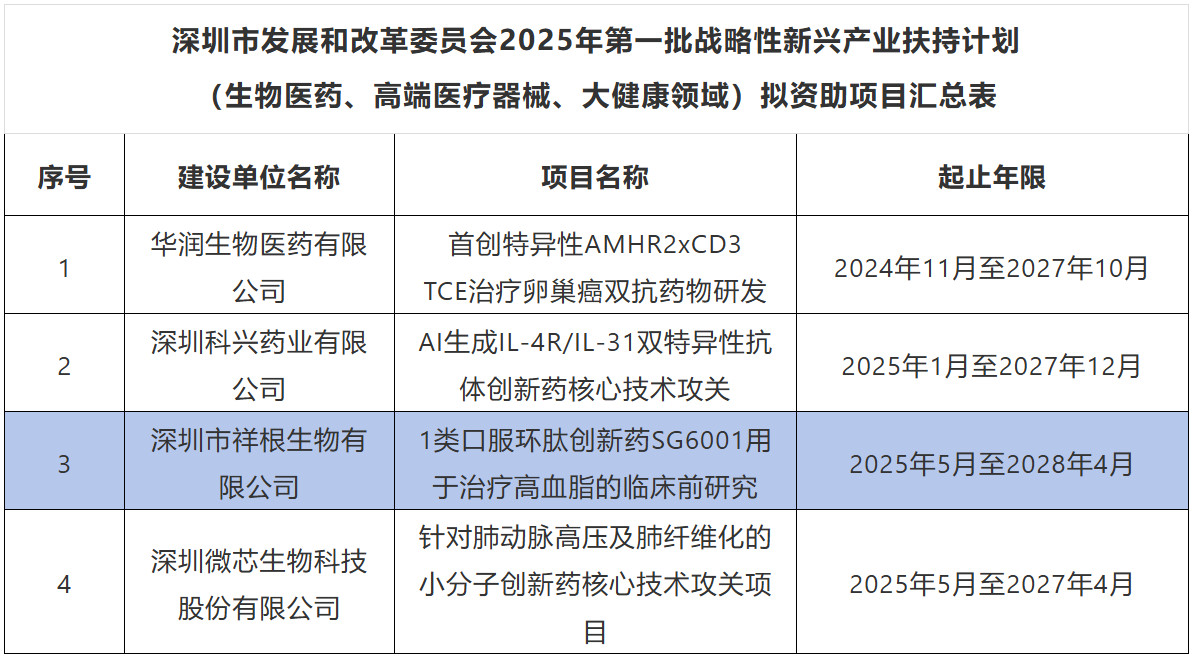
Recently, the Shenzhen Municipal Development and Reform Commission announced the list of proposed projects for the 2025 first batch of strategic emerging industries support plan. Sungening Biotechnology's independently developed oral cyclic peptide innovative drug project SG6001—"Preclinical Study of Class 1 Oral Cyclic Peptide Innovative Drug SG6001 for the Treatment of Hyperlipidemia"—has received support under the Shenzhen Municipal Development and Reform Commission's Innovative Drug Support Plan (Core Technology Breakthrough Support Special Project).
Partial screenshot of the publicized information
Sungening Biotech's innovative oral cyclic peptide drug SG6001 has received support from Shenzhen's strategic emerging industries. Its project is in line with the direction of strategic emerging industries, and Sungening Biotech's oral cyclic peptide technology is in line with the development trend of high-end and precision biopharmaceuticals, reflecting the forward-looking nature of technology and industrial driving force. At the same time, it also reflects the innovative strength and strategic value of Sungening Biotechnology in the field of biomedicine, which is an important milestone for the company to achieve technological breakthroughs and accelerate the industrialization process.
The historical opportunity of oral cyclic peptide drugs
In 2024, the global market size of cyclic peptides will exceed 4.7 billion US dollars, with oral formulations accounting for 38% (1.8 billion US dollars), marking the critical point of the industry's explosive growth. Phase III data of Merck MK-0616 (oral PCSK9 inhibitor) showed that once daily administration can reduce LDL-C by 55%, confirming the feasibility of oral cyclic peptides in the field of chronic diseases. The 79% PASI 90 response rate demonstrated by Johnson&Johnson Icotrokinra (oral IL-23R antagonist) in stage III psoriasis suggests that it may disrupt the market landscape of traditional biologics.
With the maturity of AI driven cyclic peptide design platforms and new delivery technologies, oral cyclic peptides are transitioning from "alternative injections" to "pioneering therapies". Among the top 20 pharmaceutical companies in the world by 2025, 17 have already laid out oral cyclic peptide pipelines. This field will not only reshape the billion dollar chronic disease treatment market, but may also usher in a new era of "oral macromolecular drugs".
Billion level lipid-lowering drugs blue ocean market
China has become one of the countries with the heaviest burden of hyperlipidemia in the world, with over 400 million patients and an adult prevalence rate of over 40%. The mortality rate of cardiovascular and cerebrovascular diseases continues to rise, and the trend of younger onset age is significant. The large patient base and severe disease burden have raised higher requirements for compliance, safety, and accessibility of lipid-lowering therapy.
From a global market perspective, the lipid-lowering drug track is accelerating towards innovative therapy iteration: it is expected that the global market size will reach 46.58 billion US dollars by 2033, with new drugs such as PCSK9 inhibitors having a compound annual growth rate of over 25% due to their strong lipid-lowering advantages. However, such innovative therapies rely heavily on injection therapy, and the convenience of long-term medication is insufficient, greatly limiting market penetration (especially for cardiovascular high-risk populations that require lifelong management).
In this context, oral cyclopeptide drugs show unique value: on the one hand, their oral dosage forms can cover more than 200 million cardiovascular high-risk groups requiring long-term medication (such as elderly patients with diabetes and kidney disease), avoiding the problem of injection compliance; On the other hand, compared to traditional statins (which 30% of patients are forced to discontinue due to side effects such as liver enzyme injury and muscle toxicity), oral cyclic peptide drugs have higher safety and potential plaque reversal ability, which can better meet clinical unmet needs.
At present, the track has shown clear market potential - the market size of oral cyclic peptide drugs is expected to be between 8 billion yuan and 12 billion US dollars by 2028. With in-depth research and development, such drugs are expected to further optimize their efficacy and economy in the future, which will not only become an important weapon for cardiovascular disease prevention and control, but also promote the industry to upgrade from "passive lipid control" to "active prevention" mode, providing key support for reducing the incidence rate and mortality of cardiovascular and cerebrovascular diseases in China.
If the innovative oral cyclic peptide drug SG6001 is successfully developed, it will greatly promote the upgrading of China's biopharmaceutical industry structure and provide a safer, more efficient, and economical new treatment plan for hyperlipidemia and cardiovascular diseases.




